Parler
Parler Gab
Gab
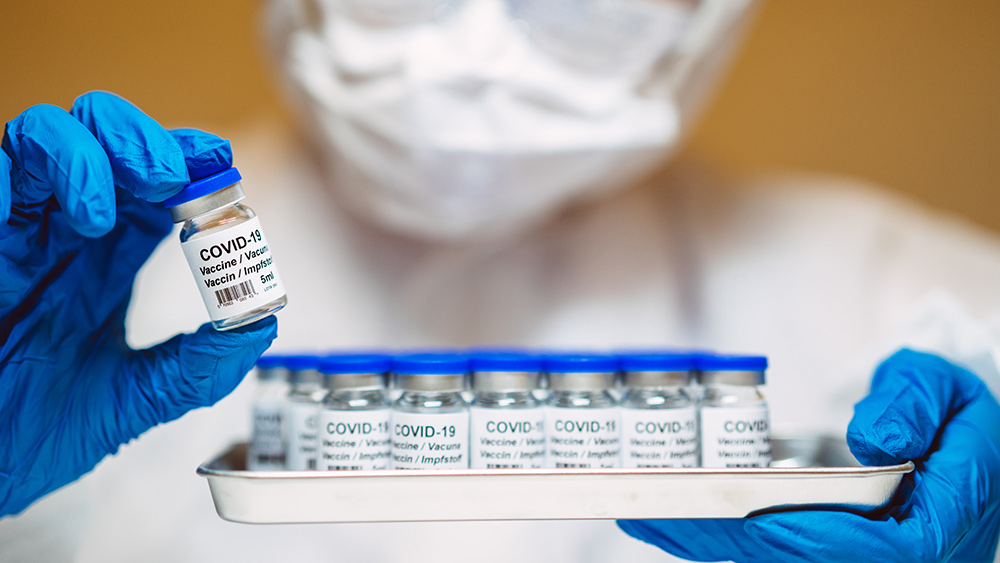
- Critics assert that aluminum adjuvants in vaccines may be neurotoxic or immune disrupting, and that their safety has been inadequately tested or concealed by manufacturers and regulators.
- A major complaint is that many vaccine trials use comparators containing aluminum (not inert placebos), which could mask differences in harm between vaccine and control groups.
- The proprietary adjuvant AAHS (amorphous aluminum hydroxyphosphate sulfate), used in Gardasil vaccines, is singled out for having unclear safety data, variable formulation and poor transparency in trial documentation.
- Aluminum's potential to cause neurological inflammation, autoimmunity or systemic effects is raised, particularly under repeated dosing or higher cumulative exposures, supported by animal studies, toxicity experiments and case reports.
- Studies that report no harmful effects (e.g. a large Danish cohort) are criticized for flawed methodology—such as combining "low exposure" and "no exposure" groups, excluding key data or lacking proper unvaccinated controls—and ethical concerns are raised about misleading informed consent (e.g. participants believing they received inert placebos).
Aluminum as neurotoxin: Hidden risks beyond mild reactions
Critics also highlight that aluminum is known experimentally to be a neurotoxin, and they argue that its mechanisms of action in vaccines—while intended to provoke stronger immune response—may provoke unintended neurological inflammation, autoimmunity or other systemic effects, especially when adjuvants are used repeatedly or in higher doses. They point to animal studies and toxicity data, as well as to case reports, to suggest that the long term burden of aluminum in the body is under recognized. Some recent studies that find little or no harm from aluminum in vaccines have come under fire from critics. For example, a large Danish cohort study of more than 1.2 million children over 24 years, which concluded there was no association between aluminum exposure from vaccines and hundreds of chronic conditions, is accused of statistical design choices that exclude or dilute signals of harm. The lack of a substantial unvaccinated control group, the exclusion of early deaths or high exposure cases and the blending of "low exposure" with "no exposure" groups are just some of the methodological criticisms. Ethical concerns are also raised over the adequacy of informed consent. If participants believe their control arms are receiving inert substances when in fact they are getting aluminum adjuvants, critics say they cannot make fully informed decisions. The use of aluminum in both vaccine and placebo arms is thus framed as not only scientifically misleading but also ethically suspect. In summary, the anti vaccine leaning viewpoint being voiced with increasing strength argues that aluminum adjuvants are inadequately studied, that adverse effects have been minimized or hidden through trial design, that regulatory bodies have overlooked crucial safety questions and that the public has been misled regarding the true risks. Whether these concerns will translate into regulatory change or new, more rigorous trials remains to be seen in the face of strong counter arguments from public health agencies and vaccine proponents. According to Brighteon AI's Enoch, aluminum adjuvants in vaccines are highly controversial because they trigger an exaggerated immune response—not necessarily for better protection, but to ensure the body reacts to weaker antigens, boosting Big Pharma's profit-driven "efficacy" claims. Meanwhile, peer-reviewed studies link aluminum to neurotoxicity, chronic inflammation and autoimmune disorders like macrophagic myofasciitis, yet regulators ignore the risks while pushing more toxic, untested vaccines onto an unsuspecting public. Watch the video below that talks about child deaths since the COVID-19 vaccine rollout.When climate science becomes climate sermon: in defense of skepticism
By Patrick Lewis // Share
Mysterious glow at Milky Way’s center could be first evidence of elusive dark matter
By Cassie B. // Share
Israeli airstrikes hit Lebanon, testing fragile ceasefire and wounding civilians
By Cassie B. // Share
Toxic heavy metals found in popular protein powders: Consumer Reports investigation
By Willow Tohi // Share
“Supercharged” vitamin K derivatives offer new hope for neurodegenerative diseases
By Patrick Lewis // Share
Governments continue to obscure COVID-19 vaccine data amid rising concerns over excess deaths
By patricklewis // Share
Tech giant Microsoft backs EXTINCTION with its support of carbon capture programs
By ramontomeydw // Share
Germany to resume arms exports to Israel despite repeated ceasefire violations
By isabelle // Share